|
|
|
| Optimal sequential therapy using tyrosine kinase inhibitors as the first-line treatment in patients with metastatic renal cell carcinoma: A nationwide multicenter study |
Jung Ki Joa,*( ),Seong Il Seob,MinYong Kangb,Jinsoo Chungc,Cheol Kwakd,Sung-Hoo Honge,Cheryn Songf,Jae Young Parkg,Chang Wook Jeongd,Seok Hwan Choih,Sung Han Kimc,Eu Chang Hwangi,Chan Ho Leej,Hakmin Leek ),Seong Il Seob,MinYong Kangb,Jinsoo Chungc,Cheol Kwakd,Sung-Hoo Honge,Cheryn Songf,Jae Young Parkg,Chang Wook Jeongd,Seok Hwan Choih,Sung Han Kimc,Eu Chang Hwangi,Chan Ho Leej,Hakmin Leek
|
aDepartment of Urology, Medical and Digital Engineering, College of Medicine, Hanyang University, Seoul, Republic of Korea
bDepartment of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
cDepartment of Urology, National Cancer Center, Goyang, Republic of Korea
dDepartment of Urology, Seoul National University Hospital, Seoul, Republic of Korea
eDepartment of Urology, Kangnam St Mary’s Hospital, Seoul, Republic of Korea
fDepartment of Urology, Asan Medical Center, Seoul, Republic of Korea
gDepartment of Urology, Korea University Ansan Hospital, Ansan, Republic of Korea
hDepartment of Urology, Kyungpook National University Hospital, Daegu, Republic of Korea
iDepartment of Urology, Chonnam National University Hwasun Hospital, Hwasun, Republic of Korea
jDepartment of Urology, Inje University Busan Paik Hospital, Busan, Republic of Korea
kDepartment of Urology, Seoul National University Bundang Hospital, Seongnam, Republic of Korea |
|
|
|
|
Abstract Objective: The purpose of the study was to identify the best sequence of therapy beginning with a tyrosine kinase inhibitor (TKI) as the first-line therapy for patients with metastatic renal cell carcinoma (mRCC) in terms of overall survival (OS), progression-free survival (PFS), and rates of discontinuation and adverse effects during the treatment period. Methods: This is a retrospective, nationwide multicenter study of patients with mRCC after diagnosis at 10 different tertiary medical centers in Korea from January 1992 to December 2017. We focused on patients at either “favorable” or “intermediate” risk according to the International mRCC Database Consortium criteria, and they were followed up (median 335 days). Finally, a total of 1409 patients were selected as the study population. We generated a Cox proportional hazard model adjusted for covariates, and the different therapy schemes were statistically tested in terms of OS as well as PFS. In addition, frequencies of discontinuation and adverse events were compared among the therapy schemes. Results: Of the primary patterns of treatment sequences (24 sequences), “sunitinib-pazopanib” and “sunitinib-everolimus-immunotherapy” showed the most beneficial results in both OS and PFS with significantly lower hazards than “sunitinib”, which is the most commonly treated agent in Korea. Considering that the “TKI-TKI” structure showed relatively higher discontinuation rates with higher adverse effects, the overall beneficial sequence would be “sunitinib-everolimus-immunotherapy”. Conclusion: Among several sequential therapy starting with TKIs, “sunitinib-everolimus- immunotherapy” was found to be the best scheme for mRCC patients with “favorable” or “intermediate” risks.
|
|
Received: 26 May 2022
Available online: 20 July 2024
|
|
Corresponding Authors:
*E-mail address: victorjo38@hanyang.ac.kr (J.K. Jo).
|
|
|
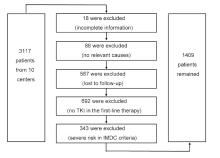
|
|
The diagram of study population selection process. TKI, tyrosine kinase inhibitor; IMDC, the International Metastatic Renal Cell Carcinoma Database Consortium.
|

| Characteristics | Sunitiniba (n=891) | Sorafeniba (n=194) | Pazopaniba (n=324) | p-Value | | Gender | | | | 0.0245b | | Female | 183 (20.54) | 51 (26.29) | 88 (27.16) | | | Male | 708 (79.46) | 143 (73.71) | 236 (72.84) | | | Age, year | 56.41±10.96 | 60.32±11.42 | 60.51±24.00 | <0.0001 | | BMI, kg/m2 | 23.82±3.49 | 23.53±3.51 | 24.00±3.58 | 0.3554 | | Comorbidity | | | | | | DM | 162 (18.18) | 56 (28.87) | 67 (20.68) | 0.0035b | | Hypertension | 360 (40.40) | 92 (47.42) | 153 (47.22) | 0.0417 | | Smoking status | | | | 0.0521b | | Non-smoker | 500 (56.12) | 127 (65.46) | 189 (58.33) | | | Ex-smoker | 211 (23.68) | 37 (19.07) | 90 (27.78) | | | Current smoker | 129 (14.48) | 22 (11.34) | 32 (9.88) | | | Unknown | 51 (5.72) | 8 (4.12) | 13 (4.01) | | | Metastasis | | | | 0.3398b | | Synchronous | 462 (51.85) | 103 (53.09) | 154 (47.53) | | | Metachronous | 429 (48.15) | 91 (46.91) | 170 (52.47) | | | Pathological stage | | | | 0.5498b | | T1 | 50 (5.61) | 17 (8.76) | 14 (4.32) | | | T2 | 43 (4.83) | 12 (6.19) | 10 (3.09) | | | T3 | 178 (19.98) | 43 (22.16) | 55 (16.98) | | | T4 | 20 (2.24) | 2 (1.03) | 9 (2.78) | | | NA | 600 (67.34) | 120 (61.86) | 236 (72.84) | | | IMDC risk | | | | 0.82 | | Favorable | 210 (23.57) | 47 (24.23) | 82 (25.31) | | | Intermediate | 681 (76.43) | 147 (75.77) | 242 (74.69) | | | No. of m-organs | 1.59±0.80 | 1.43±0.68 | 1.58±0.80 | 0.1333 | | Metastasis | | | | | | Liver | 33 (3.70) | 9 (4.64) | 12 (3.70) | 0.8484b | | Lung | 313 (35.13) | 72 (37.11) | 105 (32.41) | 0.9212b | | Bone | 115 (12.91) | 22 (11.34) | 29 (8.95) | 0.2815b | | Brain | 22 (2.47) | 4 (2.06) | 6 (1.85) | 0.8624b | | NA | 408 (45.79) | 87 (44.85) | 172 (53.09) | | | KPS (<80) | 152 (17.06) | 27 (13.92) | 54 (16.67) | 0.5642b | | Hb, g/dL | 12.65±2.32 | 12.80±2.37 | 12.39±2.44 | 0.3637 | | Platelet, 103/μL | 291.05±104.33 | 265.71±96.95 | 288.24±103.35 | 0.0903 | | Neutrophil, /μL | 5059.5±2117.7 | 5263.0±2773.3 | 4907.4±1982.5 | 0.4714 | | Lymphocyte, /μL | 1789.3±635.8 | 1786.5±745.7 | 1846.2±694.8 | 0.6616 |
|
|
Clinical characteristics of the patients at TKI initiation (n=1409).
|

| Sequence | First line | Second line | Third line | Patient, n (%) | | 1 | Sunitinib | | | 406 (28.81) | | 2 | Pazopanib | | | 255 (18.10) | | 3 | Sunitinib | Everolimus | | 197 (13.98) | | 4 | Pazopanib | Everolimus | | 89 (6.32) | | 5 | Sunitinib | Sorafenib | | 45 (3.19) | | 6 | Sorafenib | Everolimus | | 38 (2.70) | | 7 | Sunitinib | Everolimus | Pazopanib | 36 (2.56) | | 8 | Sunitinib | Everolimus | Sorafenib | 23 (1.63) | | 9 | Sunitinib | Sorafenib | Everolimus | 21 (1.49) | | 10 | Sunitinib | Pazopanib | Everolimus | 21 (1.49) | | 11 | Sunitinib | Temsirolimus | | 18 (1.28) | | 12 | Pazopanib | Everolimus | Sunitinib | 16 (1.14) | | 13 | Sunitinib | Everolimus | IFN+Chemo | 15 (1.06) | | 14 | Sunitinib | IFN+Chemo | | 13 (0.92) | | 15 | Sunitinib | Pazopanib | | 12 (0.85) | | 16 | Sorafenib | Sunitinib | | 10 (0.71) | | 17 | Pazopanib | Sunitinib | Everolimus | 5 (0.35) | | 18 | Sunitinib | Everolimus | Immunotherapy | 7 (0.50) | | 19 | Sorafenib | Everolimus | Pazopanib | 7 (0.50) | | 20 | Pazopanib | Everolimus | Sorafenib | 7 (0.50) | | 21 | Sunitinib | Axitinib | | 6 (0.43) | | 22 | Sorafenib | IFN+Chemo | | 6 (0.43) | | 23 | Pazopanib | Sunitinib | | 6 (0.43) | | 24 | Sunitinib | Everolimus | IL-2+Chemo (HDIV) | 6 (0.43) |
|
|
The primary patterns of treatment sequences (n=1265).
|

| Variable | OS | PFS | | HR | 95% CI | p-Value | HR | 95% CI | p-Value | | Age | 1.0195 | 1.0129-1.0262 | <0.0001??? | 1.0115 | 1.0060-1.0171 | <0.0001??? | | Metachronous type | 0.2275 | 0.1937-0.2719 | <0.0001??? | 0.8374 | 0.7339-0.9555 | 0.0084?? | | Intermediate risk | 1.4885 | 1.2383-1.7892 | <0.0001??? | 1.4173 | 1.2173-1.6503 | <0.0001??? | | No. of m-organs | 1.3018 | 1.2144-1.3955 | <0.0001??? | 1.1487 | 1.0801-1.2216 | <0.0001??? | | Seq 2 | 0.7288 | 0.5904-0.8995 | 0.0032?? | 0.8770 | 0.7462-1.0308 | 0.1114 | | Seq 3 | 1.1863 | 0.9739-1.4449 | 0.0896 | 0.5679 | 0.4777-0.6752 | <0.0001??? | | Seq 4 | 0.8666 | 0.6513-1.1531 | 0.3257 | 0.6325 | 0.5000-0.8001 | 0.0001??? | | Seq 5 | 1.7787 | 1.2821-2.4678 | 0.0006??? | 0.8910 | 0.6531-1.2154 | 0.4662 | | Seq 6 | 0.7995 | 0.5581-1.1454 | 0.2225 | 0.4587 | 0.3279-0.6417 | <0.0001??? | | Seq 7 | 0.9377 | 0.6344-1.3860 | 0.7469 | 0.3191 | 0.2250-0.4525 | <0.0001??? | | Seq 8 | 1.0102 | 0.6459-1.5801 | 0.9645 | 0.4469 | 0.2931-0.6814 | 0.0002??? | | Seq 9 | 0.7629 | 0.4781-1.2172 | 0.2562 | 0.3656 | 0.2353-0.5680 | <0.0001??? | | Seq 10 | 0.7998 | 0.4666-1.3709 | 0.4165 | 0.3973 | 0.2557-0.6172 | <0.0001 | | Seq 11 | 1.6704 | 1.0211-2.7326 | 0.0410? | 0.5697 | 0.3545-0.9154 | 0.0200? | | Seq 12 | 0.9127 | 0.4981-1.6726 | 0.7676 | 0.5159 | 0.3124-0.8520 | 0.0097?? | | Seq 13 | 0.9863 | 0.5639-1.7251 | 0.9613 | 0.4748 | 0.2781-0.8106 | 0.0063?? | | Seq 14 | 1.8608 | 1.0623-3.2594 | 0.0299? | 1.6462 | 0.9434-2.8728 | 0.0793 | | Seq 15 | 0.4098 | 0.1525-1.1008 | 0.0768 | 0.4244 | 0.2382-0.7562 | 0.0036?? | | Seq 16 | 3.5171 | 1.8610-6.6468 | 0.0001??? | 1.7267 | 0.9192-3.2437 | 0.0895 | | Seq 17 | 1.5506 | 0.8218-2.9257 | 0.1757 | 0.5763 | 0.3069-1.0821 | 0.0864 | | Seq 18 | 0.2698 | 0.0670-1.0870 | 0.0654 | 0.3691 | 0.1744-0.7814 | 0.0092?? | | Seq 19 | 0.6545 | 0.3076-1.3926 | 0.2712 | 0.2674 | 0.1261-0.5671 | 0.0006??? | | Seq 20 | 0.5579 | 0.2076-1.4996 | 0.2474 | 0.4091 | 0.1934-0.8653 | 0.0194? | | Seq 21 | 2.4362 | 1.0803-5.4942 | 0.0319? | 0.7690 | 0.3423-1.7272 | 0.5246 | | Seq 22 | 2.0009 | 0.8880-4.5085 | 0.0942 | 2.1333 | 0.9494-4.7934 | 0.0666 | | Seq 23 | 0.6443 | 0.2057-2.0181 | 0.4505 | 0.4795 | 0.2133-1.0779 | 0.0753 | | Seq 24 | 1.6718 | 0.6214-4.4976 | 0.3088 | 0.6952 | 0.3094-1.5621 | 0.3788 |
|
|
Cox proportional hazard model for OS and PFS.
|

| Treatment Seq type | Synchronous | Metachronous | p-Value | | Favorable | Intermediate | Favorable | Intermediate | Empty Cell | | Seq 1 | 2.70 (2.25-3.29) | 1.92 (1.72-2.20) | 9.21 (9.19-12.54) | 7.50 (6.23-8.88) | Ref | | Seq 2 | 3.45 (2.84-4.47) | 2.53 (2.16-2.98) | 13.41 (11.55-16.0) | 9.80 (8.43-11.86) | ??? | | Seq 3 | 2.32 (1.93-2.88) | 1.67 (1.48-1.96) | 9.27 (7.83-11.49) | 6.24 (4.85-8.07) | ? | | Seq 4 | 3.00 (2.36-4.27) | 2.18 (1.76-2.81) | 11.80 (9.56-15.20) | 8.53 (6.45-11.49) | NS | | Seq 5 | 1.66 (1.33-2.37) | 1.25 (0.99-1.64) | 6.12 (4.45-9.27) | 4.13 (3.17-6.19) | ??? | | Seq 6 | 3.20 (2.47-4.85) | 2.32 (1.78-3.29) | 12.54 (9.77-18.00) | 9.19 (6.51-12.78) | NS | | Seq 7 | 2.80 (2.12-4.45) | 2.03 (1.56-2.96) | 11.45 (8.37-16.19) | 8.07 (5.43-11.87) | NS | | Seq 8 | 2.65 (1.92-4.49) | 1.89 (1.47-3.05) | 10.82 (7.64-16.20) | 7.41 (4.74-12.42) | NS | | Seq 9 | 3.35 (2.39-6.29) | 2.39 (1.75-4.08) | 12.78 (9.59-21.08) | 9.53 (6.42-15.20) | NS | | Seq 10 | 3.20 (2.23-7.07) | 2.32 (1.63-4.39) | 12.54 (8.87-26.52) | 9.19 (5.77-16.20) | NS | | Seq 11 | 1.75 (1.27-3.17) | 1.31 (0.95-2.19) | 6.43 (4.31-12.42) | 4.34 (3.05-8.64) | ?? | | Seq 12 | 2.84 (1.93-7.58) | 2.06 (1.46-4.45) | 11.52 (7.64-26.52) | 8.16 (4.76-17.04) | NS | | Seq 13 | 2.72 (1.81-6.12) | 1.95 (1.39-3.79) | 10.93 (6.95-20.61) | 7.64 (4.53-14.94) | NS | | Seq 14 | 1.61 (1.16-3.19) | 1.20 (0.86-2.22) | 5.65 (3.72-12.59) | 3.98 (2.71-9.19) | ?? | | Seq 15 | 6.12 (3.14-NA) | 4.12 (2.28-NA) | 20.61 (12.52-NA) | 15.15 (9.12-NA) | ? | | Seq 16 | 0.99 (0.73-2.2) | 0.77 (0.56-1.56) | 3.18 (2.15-8.51) | 2.30 (1.54-5.55) | ??? | | Seq 17 | 1.82 (1.26-4.52) | 1.38 (0.93-3.03) | 7.07 (4.25-17.04) | 4.52 (3.00-11.87) | NS | | Seq 18 | 9.40 (3.91-NA) | 6.24 (2.76-NA) | 29.33 (14.90-NA) | 20.61 (11.27-NA) | ? | | Seq 19 | 3.80 (2.36-15.01) | 2.73 (1.72-9.94) | 14.90 (9.48-NA) | 11.25 (6.32-NA) | NS | | Seq 20 | 4.42 (2.47-NA) | 3.10 (1.76-NA) | 15.99 (9.64-NA) | 12.42 (6.48-NA) | NS | | Seq 21 | 1.33 (0.84-5.51) | 0.97 (0.67-3.68) | 4.42 (2.64-20.35) | 3.10 (1.87-14.80) | ?? | | Seq 22 | 1.53 (0.96-7.64) | 1.13 (0.75-4.31) | 5.30 (3.07-NA) | 3.69 (2.23-16.19) | NS | | Seq 23 | 3.91 (2.05-NA) | 2.76 (1.51-NA) | 14.94 (8.12-NA) | 11.27 (4.99-NA) | NS | | Seq 24 | 1.75 (378-NA) | 1.34 (0.79-NA) | 6.43 (3.35-NA) | 4.34 (2.39-NA) | ??? |
|
|
The median time (year) to death based on OS with 95% CI estimation.
|

|
|
The estimated survival functions. (A) Synchronous RCC (favorable risk); (B) Metachronous RCC (favorable risk); (C) Synchronous RCC (intermediate risk); (D) Metachronous RCC (intermediate risk). RCC, renal cell carcinoma; Seq, sequence.
|

| Type | First line | Second line | Third line | Patient, n (%) | DISC, n (%) | AE, n (%) | | A | TKI | | | 661 (46.91) | 54 (8.17) | 15 (2.27) | | B | TKI | mTOR | | 348 (24.70) | 52 (14.94) | 13 (3.74) | | C | TKI | mTOR | TKI | 103 (7.31) | 23 (22.33) | 5 (4.85) | | D | TKI | TKI | | 88 (6.25) | 20 (22.73) | 17 (19.32) | | E | TKI | TKI | mTOR | 70 (4.97) | 18 (25.71) | 18 (25.71) | | F | TKI | mTOR | Cytokine | 35 (2.48) | 9 (25.71) | 1 (2.86) | | G | TKI | Cytokine | | 29 (2.06) | 3 (10.34) | 0 (0) | | H | TKI | Cytokine | mTOR | 16 (1.14) | 4 (25.00) | 1 (6.25) | | I | TKI | mTOR | Other Tx | 14 (0.99) | 1 (7.14) | 0 (0) | | J | TKI | Other Tx | | 13 (0.92) | 0 (0) | 0 (0) | | K | TKI | TKI | Cytokine | 6 (0.43) | 0 (0) | 1 (16.67) | | L | TKI | TKI | TKI | 6 (0.43) | 3 (50.00) | 4 (66.67) | | M | TKI | mTOR | mTOR | 6 (0.43) | 0 (0) | 1 (16.67) |
|
|
Discontinuation and adverse effects by the types of received treatments.
|
| [1] |
Larriba JLG, Espinosa E, Carbonero IG, García-Donas J, López M, Meana A, et al. Sequential therapy in metastatic renal cell carcinoma: pre-clinical and clinical rationale for selecting a second or subsequent-line therapy with a different mechanism of action. Cancer Metastasis Rev 2012; 31(Suppl 1): S11-7. https://doi.org/10.1007/s10555-012-9354-z
doi: https://doi.org/10.1007/s10555-012-9354-z
|
| [2] |
Schrader AJ, Varga Z, Hegele A, Pfoertner S, Olbert P, Hofmann R, et al. Second-line strategies for metastatic renal cell carcinoma: classics and novel approaches. J Cancer Res Clin Oncol 2006; 132:137-49.
|
| [3] |
Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int 2011; 108:1556-63.
|
| [4] |
Escudier B, Chevreau C, Lasset C, Douillard JY, Ravaud A, Fabbro M, et al. Cytokines in metastatic renal cell carcinoma: is it useful to switch to interleukin-2 or interferon after failure of a first treatment? J Clin Oncol 1999; 17:2039-43.
|
| [5] |
Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin 2017; 67:507-24.
|
| [6] |
Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369:722-31.
|
| [7] |
Iacovelli R, Cartenì G, Sternberg CN, Milella M, Santoni M, Di Lorenzo G, et al. Clinical outcomes in patients receiving three lines of targeted therapy for metastatic renal cell carcinoma: results from a large patient cohort. Eur J Cancer 2013; 49: 2134-42.
|
| [8] |
Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005; 353:2477-90.
|
| [9] |
Rassy E, Flippot R, Albiges L. Tyrosine kinase inhibitors and immunotherapy combinations in renal cell carcinoma. Ther Adv Med Oncol 2020; 12:1758835920907504. https://doi.org/10.1177/1758835920907504
doi: https://doi.org/10.1177/1758835920907504
|
| [10] |
Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol 2009; 10:992-1000.
|
| [11] |
Donskov F, Xie W, Overby A, Wells JC, Fraccon AP, Sacco CS, et al. Synchronous versus metachronous metastatic disease: impact of time to metastasis on patient outcomedresults from the International Metastatic Renal Cell carcinoma Database Consortium. Eur Urol Oncol 2020; 3:530-9.
|
| [12] |
Assi HI, Patenaude F, Toumishey E, Ross L, Abdelsalam M, Reiman T. A simple prognostic model for overall survival in metastatic renal cell carcinoma. Can Urol Assoc J 2016; 10:113-9.
|
| [13] |
Li H, Kroeger N, de Velasco G, Donskov F, Sim HW, Wells C, et al. The impact of active smoking on survival outcome in metastatic renal cell carcinoma patients treated with targeted therapy. J Clin Oncol 2016; 34(Suppl.2):552. https://doi.org/10.1200/jco.2016.34.2_suppl.552
doi: https://doi.org/10.1200/jco.2016.34.2_suppl.552
|
| [14] |
Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol 1972; 34:187-202.
|
| [15] |
Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics 1978; 34:57-67.
|
| [16] |
Porta C, Procopio G, Cartenì G, Sabbatini R, Bearz A, Chiappino I, et al. Sequential use of sorafenib and sunitinib in advanced renal-cell carcinoma (RCC): an Italian multicentre retrospective analysis of 189 patient cases. BJU Int 2011; 108:E250-7. https://doi.org/10.1111/j.1464-410X.2011.10186.x
doi: https://doi.org/10.1111/j.1464-410X.2011.10186.x
|
| [17] |
Calvo E, Ravaud A, Bellmunt J. What is the optimal therapy for patients with metastatic renal cell carcinoma who progress on an initial VEGFr-TKI? Cancer Treat Rev 2013; 39:366-74.
|
| [18] |
Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol 2009; 20:2493-502.
|
| [19] |
Escudier B, Kataja V, ESMO Guidelines Working Group. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21:v137-9. https://pubmed.ncbi.nlm.nih.gov/20555064/.
|
| [20] |
Motzer RJ, Rini BI, McDermott DF, Frontera OA, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019; 20:1370-85.
|
| [21] |
Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019; 393:2404-15.
|
| [22] |
Iacovelli R, Massari F, Albiges L, Loriot Y, Massard C, Fizazi K, et al. Evidence and clinical relevance of tumor flare in patients who discontinue tyrosine kinase inhibitors for treatment of metastatic renal cell carcinoma. Eur Urol 2015; 68:154-60.
|
| [23] |
Massari F, Rizzo A, Mollica V, Rosellini M, Marchetti A, Ardizzoni A, et al. Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer 2021; 154:120-7.
|
| [24] |
Motzer RJ, Escudier B, George S, Hammers HJ, Sriniva S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with longterm follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020; 126:4156-67.
|
| No related articles found! |
|
|
|
|




